Lecture 20: Bioimaging II¶
CBIO (CSCI) 4835/6835: Introduction to Computational Biology
Overview and Objectives¶
Today, we'll wrap up our module on image processing with some more in-depth examples of how to analyze biological images. In particular, we'll look at video data, arguably an important format for when you want to study some kind of system with a temporal component. By the end of this lecture, you should be able to
- Understand how thresholding and segmentation are still relevant with video
- Apply cross-entropy thresholding for adaptive segmentation
- Read raw video formats into Python NumPy arrays using OpenCV
- Use previous techniques to "preprocess" videos and identify potential objects
- Implement a "centroid tracking" method to register and follow objects
Part 1: Improved Segmentation¶
In the previous lecture, we discussed myriad techniques for processing images to try and find "objects" of interest in the images.
To a certain extent, all these approaches relied on the fundamental concept of a "filter", which entailed two components:
- The image itself (a large matrix), and
- a convolutional filter, or kernel (a small matrix)
These two components are multiplied together element-wise and summed into one number, in a kind of "matrix dot-product" (with the exception of "non-linear" filters, like median or maximum).
From the concept of convolutions, we discussed several other strategies:
- Thresholding, which can take the form of hard thresholds (pixel values) or more adaptive approaches (means or standard deviations)
- Edge detection, which looks for so-called "image derivatives" that indicate edges, as implemented in the Canny Edge Detector, and taken to its logical conclusion with skeletonization
- Morphology, such as erosion and dilation, which can smooth out rough edges, fill in pixel gaps, and eliminate small flecks of noise
- Segmentation, such as Seeded Watershed, which uses the idea of a contour or elevation map being flooded with water from a set of initial "seeds"
Anything I'm forgetting?
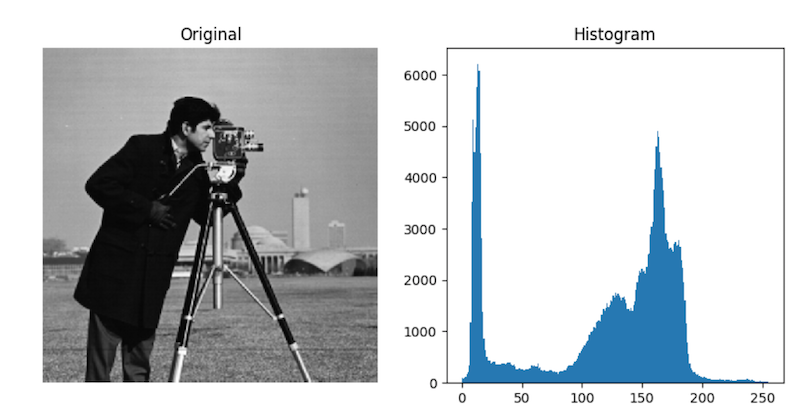
Remember our good friend, heat shock protein 27?
%matplotlib inline
import matplotlib.pyplot as plt
import skimage.io as skio
img = skio.imread("ComputerVision/image1.png")
f = plt.figure(figsize = (18, 9))
f.add_subplot(1, 3, 1)
plt.title("Actin")
plt.imshow(img[:, :, 0], cmap = "Reds")
f.add_subplot(1, 3, 2)
plt.title("HSP")
plt.imshow(img[:, :, 1], cmap = "Greens")
f.add_subplot(1, 3, 3)
plt.title("DAPI")
plt.imshow(img[:, :, 2], cmap = "Blues")
<matplotlib.image.AxesImage at 0x8135df048>
We used a combination of strategies to try and identify the cells containing the most HSP27.
# HSP27 channel only
hsp = img[:, :, 1]
plt.imshow(hsp, cmap = "gray")
<matplotlib.image.AxesImage at 0x813733898>
Remember our results? They weren't very good...
hsp_bin = hsp > hsp.mean()
f = plt.figure(figsize = (16, 8))
f.add_subplot(1, 2, 1)
plt.title("Grayscale")
plt.imshow(hsp, cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Thresholded")
plt.imshow(hsp_bin, cmap = "gray")
<matplotlib.image.AxesImage at 0x813786e10>
Let's try a different thresholding approach.
import skimage.filters as skfi
hsp_min = hsp > skfi.threshold_minimum(hsp)
f = plt.figure(figsize = (16, 8))
f.add_subplot(1, 3, 1)
plt.title("Grayscale")
plt.imshow(hsp, cmap = "gray")
f.add_subplot(1, 3, 2)
plt.title("Thresholded")
plt.imshow(hsp_bin, cmap = "gray")
f.add_subplot(1, 3, 3)
plt.title("Minimum")
plt.imshow(hsp_min, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c15d45550>
The "minimum" threshold takes advantage of the fact that a lot of images have histograms that are bimodal, and can therefore be sensitive to first-order statistics like mean. It repeatedly runs smoothing filters over the image until only two peaks remain in the histogram, then sets the threshold to be the minimum value between them.
In a similar vein, the Otsu threshold tries to find an "optimal" separation point between the two peaks in the pixel histogram: the point at which distance between pixels in the same group is larger than the distance between pixels in different groups.
hsp_otsu = hsp > skfi.threshold_otsu(hsp)
f = plt.figure(figsize = (16, 8))
f.add_subplot(1, 4, 1)
plt.title("Grayscale")
plt.imshow(hsp, cmap = "gray")
f.add_subplot(1, 4, 2)
plt.title("Thresholded")
plt.imshow(hsp_bin, cmap = "gray")
f.add_subplot(1, 4, 3)
plt.title("Minimum")
plt.imshow(hsp_min, cmap = "gray")
f.add_subplot(1, 4, 4)
plt.title("Otsu")
plt.imshow(hsp_otsu, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c160eca90>
The Otsu filter in particular seems closest to what we want. Still, it's not perfect.
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Grayscale")
plt.imshow(hsp, cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Otsu")
plt.imshow(hsp_otsu, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c163e34a8>
One way to fix this could be to manually adjust the threshold that Otsu comes back with until it "looks right".
# The "- 40" makes the threshold *lower*
hsp_otsu2 = hsp > (skfi.threshold_otsu(hsp) - 40)
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Grayscale")
plt.imshow(hsp, cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Otsu 2")
plt.imshow(hsp_otsu2, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c1661fb70>
What are the strengths and weaknesses of this approach?
Strengths
- A pixel threshold, so it's fast and simple
- Semi-intelligent, aka adaptive
- Major, major improvement in quality over the segmentation we had before
Weaknesses
- Super-restrictive (only found 3 of 5 visible cells)
- Some manual tweaking needed to get all 5 cells
One more method--the minimum cross-entropy method, initially proposed in Li et al 1992 and improved upon in Li et al 199800057-9) (was everyone here born by then?).
Put simply, this method attempts to minimize cross-entropy between the original image and its segmented (thresholded) version.
"Cross-entropy" is a fancy term for "uncertainty between two distributions" (those two distributions being: the original image, and the proposed segmented version for a given threshold value).
Basically, it's trying to find a way of thresholding the image in a way that most closely mimicks the image itself (i.e., you don't see any objects in the thresholded image that weren't there in the original image).
It works... pretty darn well:
hsp_li = hsp > skfi.threshold_li(hsp)
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Grayscale")
plt.imshow(hsp, cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Li")
plt.imshow(hsp_li, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c1662e048>
Once we have such a segmentation, recall how we can compute a lot of interesting properties about the regions--area, circumference, location, even major and minor axes.
This is great for fixed cells, or otherwise rigid objects. It certainly streamlines an experimental setup where most of the work is dedicated to counting cells.
But what about live-cell imaging? What happens when we want to follow these objects, studying how they move? Or possibly even study how cells change as they move?
Part 2: Tracking Objects¶
There's an entire area of computer vision dedicated exclusively to the problem of tracking. It's a really hard problem, and often boils down to domain-specific answers (i.e., there's no single Algorithm To Track Them All).
Intuitively, though: using the concepts of the ideas we've discussed so far, how would you build a hypothetical object tracking module?
Step 1: Identify the objects in a given frame at time $t$ (like we just did with the Li threshold).
Step 2: Compute some unique feature of each object--can you think of one?
Step 3: Repeat Steps 1 and 2 on the next frame at time $t + 1$, and try to find the unique feature of each object you found in frame $t$ now in frame $t + 1$ (this is known as alignment).
Step 4: Make frame $t + 1$ the new frame $t$, and go back to Step 1.
Toxoplasma gondii¶
T. gondii is a parasitic protozoan, and one of the most successful on the planet.
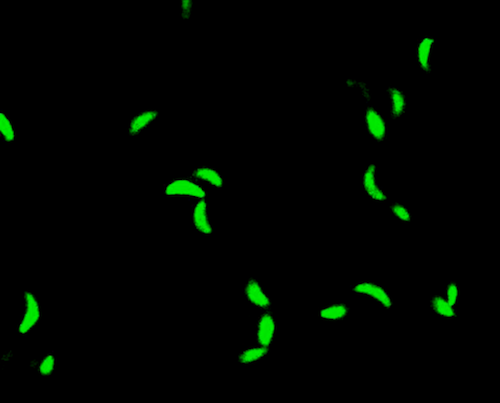
It's estimated to infect over a third of the world's population. Most of that is a latent form, held in check by healthy immune systems. But there is a risk of flare-up, and disseminated toxoplasmosis does tend to affect infants, elderly, and immunocompromised individuals.
The motility of T. gondii is directly linked to its virulence, so understanding how and why T. gondii move as they do could be key to developing targeted therapies.
from IPython.display import Video
Video("Bioimaging2/tgondii.mov")
Reading video files¶
As with images, it's still a lot like reading text files in Python.
import cv2
frames = []
cap = cv2.VideoCapture("Bioimaging2/tgondii.avi")
while cap.isOpened():
ret, frame = cap.read()
if not ret: break
gframe = cv2.cvtColor(frame, cv2.COLOR_BGR2GRAY)
frames.append(gframe)
print("Read in {} frames".format(len(frames)))
print("Each frame has height {} and width {}".format(frames[0].shape[1], frames[0].shape[0]))
Read in 900 frames Each frame has height 600 and width 600
plt.figure(figsize = (12, 6))
plt.imshow(frames[0], cmap = "Greens")
<matplotlib.image.AxesImage at 0x1c32d60f28>
Another way, which I recommend, is to use the imageio package--a lightweight Python library for reading and writing images and video (of MANY different formats!).
import imageio
cap = imageio.get_reader("Bioimaging2/tgondii.avi")
frames = []
for frame in cap:
frames.append(frame)
print("Read in {} frames".format(len(frames)))
print("Each frame has height {} and width {}".format(frames[0].shape[1], frames[0].shape[0]))
Read in 900 frames Each frame has height 600 and width 600
plt.figure(figsize = (12, 6))
plt.imshow(frames[0])
<matplotlib.image.AxesImage at 0x1c1f92cd30>
The only downside of imageio is the frames are read as RGB format:
print(frames[0].shape)
(600, 600, 3)
So these have to be converted to grayscale:
import skimage.color as skc
import skimage
import numpy as np
gray_frames = []
for frame in frames:
gray_frame = skc.rgb2gray(frame) # convert to grayscale
new_frame = skimage.img_as_ubyte(gray_frame) # convert to 0-255
gray_frames.append(new_frame)
volume = np.array(gray_frames)
/opt/python/envs/cbio/lib/python3.6/site-packages/skimage/util/dtype.py:130: UserWarning: Possible precision loss when converting from float64 to uint8 .format(dtypeobj_in, dtypeobj_out))
print(volume.shape)
(900, 600, 600)
Cross-entropy over frames¶
Your first thought for tracking the T. gondii parasites might (rightly!) be: let's just do the threshold we've been using on each frame, then try to link up the objects?
vol_bin = volume[0] > skfi.threshold_li(volume[0])
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Grayscale")
plt.imshow(volume[0], cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Li")
plt.imshow(vol_bin, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c819ed828>
bin_volume = np.array([v > skfi.threshold_li(v) for v in volume])
So... what's next?
We need to compute some kind of unique feature for each object, something that we can track between frames. Any ideas?
Centroid!
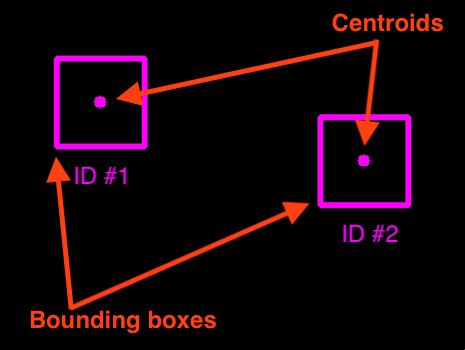
We can do this in a handful of steps (similar to what we wrote earlier):
Step 1: Find the objects and compute their centroids (Steps 1 and 2 from before).
Step 2: Compute Euclidean distance between each centroid in the current frame (at time $t + 1$) and those from the previous frame (at time $t$).
(Source: https://www.pyimagesearch.com/2018/07/23/simple-object-tracking-with-opencv/ )
Step 3: Update our records of each object in terms of their locations.
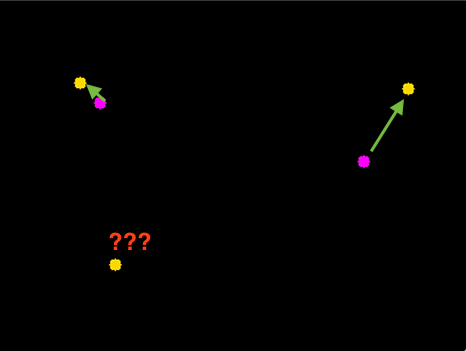
(Source: https://www.pyimagesearch.com/2018/07/23/simple-object-tracking-with-opencv/ )
Step 4: Register all the objects we're tracking (basically "have a mechanism to keep track of them"; can you think of one??).
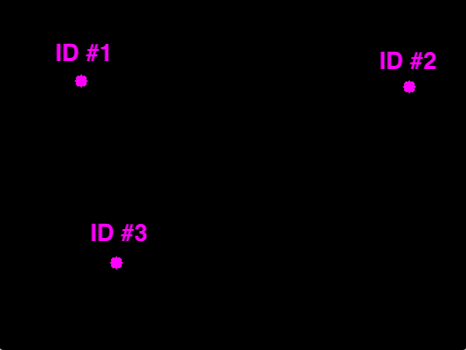
(Source: https://www.pyimagesearch.com/2018/07/23/simple-object-tracking-with-opencv/ )
Step 5: De-register any objects we've lost track of (when would this happen??).
"But," you ask, "how do we compute centroids and bounding boxes from the binary mask we saw earlier?"
Recall two fancy functions from scikit-image:
skimage.measure.label: takes a binary image and identifies all the objects (connected pixels) in the image
skimage.measure.regionprops: computes spatial properties of all the objects in an image, including (but not limited to) the centroid of each object and its bounding box
Let's give it a shot, shall we?
# First, compute the objects using the "label" function.
import skimage.measure as skms
objects, n_obj = skms.label(bin_volume[0], return_num = True)
print("Found {} objects in frame 1.".format(n_obj))
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Binary (Li)")
plt.imshow(bin_volume[0], cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Objects")
plt.imshow(objects, cmap = "gray")
Found 38 objects in frame 1.
<matplotlib.image.AxesImage at 0x1c81ce3438>
Now, let's compute the bounding boxes and centroids for each object.
props = skms.regionprops(objects)
boxes_and_centroids = np.copy(objects)
t = objects.max() + 1
for obj in props:
# Recall: http://scikit-image.org/docs/dev/api/skimage.measure.html#regionprops
min_row, min_col, max_row, max_col = obj["bbox"]
boxes_and_centroids[min_row:max_row + 1, min_col:min_col + 2] = t
boxes_and_centroids[min_row:max_row + 1, max_col - 2:max_col] = t
boxes_and_centroids[min_row:min_row + 2, min_col:max_col + 1] = t
boxes_and_centroids[max_row - 2:max_row, min_col:max_col + 1] = t
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Objects")
plt.imshow(objects, cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Centroids and Boxes")
plt.imshow(boxes_and_centroids, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c81e384a8>
You can probably already see part of the problem: multiple objects have been grouped together.
That's a common problem, and not one that is easily solved, unfortunately.
For now, we'll continue and see if we can get a decent tracking method working.
Let's look at the 10th frame (as a way of seeing a bit more motion than we would with the 2nd frame).
objects2, n_obj2 = skms.label(bin_volume[10], return_num = True)
print("Found {} objects in frame 10 (compared to {} in frame 1).".format(n_obj2, n_obj))
Found 36 objects in frame 10 (compared to 38 in frame 1).
props2 = skms.regionprops(objects2)
boxes_and_centroids2 = np.copy(objects2)
t = objects2.max() + 1
for obj in props2:
min_row, min_col, max_row, max_col = obj["bbox"]
boxes_and_centroids2[min_row:max_row + 1, min_col:min_col + 2] = t
boxes_and_centroids2[min_row:max_row + 1, max_col - 2:max_col] = t
boxes_and_centroids2[min_row:min_row + 2, min_col:max_col + 1] = t
boxes_and_centroids2[max_row - 2:max_row, min_col:max_col + 1] = t
f = plt.figure(figsize = (12, 6))
f.add_subplot(1, 2, 1)
plt.title("Frame 1")
plt.imshow(boxes_and_centroids, cmap = "gray")
f.add_subplot(1, 2, 2)
plt.title("Frame 10")
plt.imshow(boxes_and_centroids2, cmap = "gray")
<matplotlib.image.AxesImage at 0x1c81f3aa90>
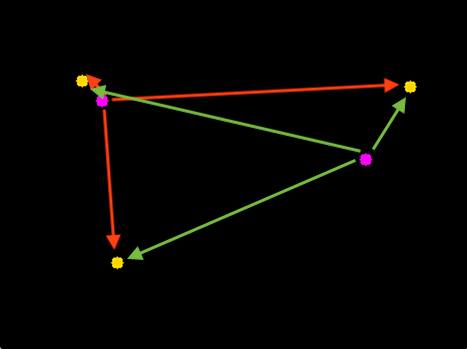
Now, the critical element: drawing equivalences between centroids of each object.
Hopefully the intuition is fairly straightforward:
- For a given centroid in frame 10, measure the distance between it and all the centroids in frame 1.
- Choose the centroid in frame 1 with the smallest distance.
- Link up those two centroids between frame 1 and frame 10, and now consider them the same object.
What are the possible complications?
- Too few / too many objects. Like we saw in the example, the number of objects we found went from 38 in frame 1 to 36 in frame 10. Two objects vanished! It could have just as easily gone the other way, too: more objects in future frames. This can happen easily given how our method of finding objects groups objects together that are touching.
- Misalignment of objects. Imagine a situation where you align one object between two frames, but that object just happened to be one that appeared in the future frame! This could set off a "misalignment cascade" where all the other objects are misaligned.
- Objects that aren't objects. Some of the "objects" in our images looked like noise--they were just tiny little things that probably shouldn't be objects in the first place.
Video("Bioimaging2/tracking.mov")
In practice, most tracking algorithms use these basic principles, with a few additions:
- Look at tracking history to "predict" future. If you're tracking a soccer or football player, you have an intuitive sense of momentum that intrinsically limits where the player could move in the next time step. We can apply the same principle to tracking to help narrow our search space for the object(s) in future frames and potentially distinguish between multiple object candidates.
- Perform segmentation and tracking simultaneously. This may seem like more work, but it dovetails off the idea of keeping track of history. If you know the direction and the shape of the object you were tracking before, you have an idea of both where it could be and what it looks like. This can really help to distinguish between multiple overlapped objects.
Regardless, it's still the same idea:
- Find the objects
- Represent each object with some unique quantitative features
- Track those features over time
Administrivia¶
- How is the project going (for those of you doing it)?
- You should have received feedback on your proposals (grades are on eLC, spoiler alert everyone got 100s)
- Presentations are Tues/Thurs April 21/23.
- Final write-ups will be due by 11:59pm on April 30 (the day of our final exam)
- Last homework assignment is due next Thursday!
Additional Resources¶
- "Simple object tracking with OpenCV", https://www.pyimagesearch.com/2018/07/23/simple-object-tracking-with-opencv/
- "Multi-object tracking with dlib", https://www.pyimagesearch.com/2018/10/29/multi-object-tracking-with-dlib/ (lots of great links here to other tracking tutorials in OpenCV)
imageio, https://imageio.github.io/